God and Nature Spring 2023
By Gregory Rummo
At the turn of the twentieth century, great advances were being made in atomic theory. In 1904, the British physicist and Nobel laureate Sir Joseph John Thomson, who had discovered the electron, proposed his “plum pudding model” of the atom, characterizing it as a smear of positive and negative charges much like a British Christmas pudding with embedded raisins (which they traditionally called plums).
Five years later, Hans Geiger and Ernest Marsden, working under the direction of Ernest Rutherford at Manchester University, devised an experiment to further elucidate atomic structure by bombarding a thin sheet of gold foil with a stream of alpha particles. Instead of the alpha particles passing straight through, some were deflected at various angles, and a few ricocheted back at 180 degrees.
This was a stunning finding. It took several more years for Rutherford to explain the observed phenomenon, which he did in a lecture he gave at Cambridge University:
It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you. On consideration, I realized that this scattering backward must be the result of a single collision, and when I made calculations I saw that it was impossible to get anything of that order of magnitude unless you took a system in which the greater part of the mass of the atom was concentrated in a minute nucleus. It was then that I had the idea of an atom with a minute massive centre carrying a charge (1).
In 1913, Danish physicist Niels Bohr presented a complete picture of the atom, what we call a planetary model, with negatively charged electrons orbiting a positively charged nucleus. He had used a combination of classical physics with the new concept of quantization proposed by German physicist Max Planck. Here is the mathematical expression that describes the Bohr equation for the one-electron hydrogen atom:
At the turn of the twentieth century, great advances were being made in atomic theory. In 1904, the British physicist and Nobel laureate Sir Joseph John Thomson, who had discovered the electron, proposed his “plum pudding model” of the atom, characterizing it as a smear of positive and negative charges much like a British Christmas pudding with embedded raisins (which they traditionally called plums).
Five years later, Hans Geiger and Ernest Marsden, working under the direction of Ernest Rutherford at Manchester University, devised an experiment to further elucidate atomic structure by bombarding a thin sheet of gold foil with a stream of alpha particles. Instead of the alpha particles passing straight through, some were deflected at various angles, and a few ricocheted back at 180 degrees.
This was a stunning finding. It took several more years for Rutherford to explain the observed phenomenon, which he did in a lecture he gave at Cambridge University:
It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you. On consideration, I realized that this scattering backward must be the result of a single collision, and when I made calculations I saw that it was impossible to get anything of that order of magnitude unless you took a system in which the greater part of the mass of the atom was concentrated in a minute nucleus. It was then that I had the idea of an atom with a minute massive centre carrying a charge (1).
In 1913, Danish physicist Niels Bohr presented a complete picture of the atom, what we call a planetary model, with negatively charged electrons orbiting a positively charged nucleus. He had used a combination of classical physics with the new concept of quantization proposed by German physicist Max Planck. Here is the mathematical expression that describes the Bohr equation for the one-electron hydrogen atom:
We accept many things without fully understanding the why or the how |
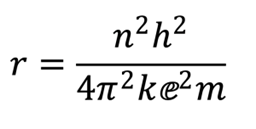
The beauty of this equation is its simplicity; n is an integer, 1, 2, 3, … and corresponds to the principal energy levels of the atom. All the other symbols are either numbers or constants that were known at the time. When this expression is solved, we obtain a picture of the hydrogen atom, its lone electron orbiting a single proton in a circular path with a radius of 0.53 Å in the ground state. Despite several flawed assumptions, Bohr’s model accurately explained observed phenomena such as the visible emission spectrum of hydrogen.
It wasn’t too long thereafter that a more complicated picture of the atom emerged as physicists embraced the idea that small particles such as electrons could be treated as if they were electromagnetic waves. In 1925, Erwin Schrödinger proposed his wave equation, a mathematical expression that causes most people (including just about every first-year chemistry student) to glaze over:
The beauty of this equation is its simplicity; n is an integer, 1, 2, 3, … and corresponds to the principal energy levels of the atom. All the other symbols are either numbers or constants that were known at the time. When this expression is solved, we obtain a picture of the hydrogen atom, its lone electron orbiting a single proton in a circular path with a radius of 0.53 Å in the ground state. Despite several flawed assumptions, Bohr’s model accurately explained observed phenomena such as the visible emission spectrum of hydrogen.
It wasn’t too long thereafter that a more complicated picture of the atom emerged as physicists embraced the idea that small particles such as electrons could be treated as if they were electromagnetic waves. In 1925, Erwin Schrödinger proposed his wave equation, a mathematical expression that causes most people (including just about every first-year chemistry student) to glaze over:
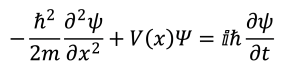
Without getting too deep into the weeds, the Schrödinger Equation is a differential equation that describes the total energy of a system as the sum of its kinetic and potential energies.
Solutions to this equation for the one-electron atom are many and no one quite knows what to make of the resulting wave functions, especially since they contain the imaginary number i. They must be further manipulated mathematically and graphed in three dimensions to reveal the shapes of the orbitals around the atom where there is a probability of finding an electron.
Looking at a mathematical expression of any phenomenon usually doesn’t paint a gratifying picture of reality. The relatively simple equation governing the physics of a watermelon undergoing acceleration due to gravity as it’s dropped from a four-story parking garage, or that of a Major League fastball hurled at 102 m.p.h., cannot compare with actually observing that watermelon impacting the pavement, or seeing the baseball as an elongated, white blur and hearing it pop into the catcher’s glove.
So, we make models, or we draw pictures to aid our understanding.
In my classroom, I illustrate the Bohr model of hydrogen by swinging a tennis ball on a string around my head. The string serves as the coulombic force of attraction that keeps the ball from flying off into space. The constant flick of my wrist supplies the energy for the centripetal force that maintains the ball’s acceleration. The solutions to the Schrödinger Equation are best demonstrated with pictures, a 3-D computer simulation, or balloons fashioned into the shapes of the various atomic orbitals.
C.S. Lewis lived during this exciting era of scientific advances. He compared the inadequacy of pictures to explain the complex mathematical expressions governing atomic theory to Christ’s death on the cross for the atonement of sin, arguing that a man’s inability to fathom completely the doctrine of salvation by faith could not be a valid argument for rejecting the Gospel.
This all makes for a beautiful science-faith integration.
Writing in Mere Christianity, Lewis says:
What [scientists] do when they want to explain the atom, or something of that sort, is to give you a description out of which you can make a mental picture. But then they warn you that this picture is not what the scientists actually believe... The pictures are there only to help you understand the formula… The thing itself cannot be pictured, it can only be expressed mathematically. We are in the same boat here. We believe that the death of Christ is just that point in history at which something absolutely unimaginable from outside shows through into our own world. And if we cannot picture even the atoms of
which our own world is built, of course we are not going to be able to picture this (2).
We accept many things without fully understanding the why or the how. Lewis offers the example of enjoying a meal without fully understanding how that food nourishes us and adds, “A man can accept what Christ has done without knowing how it works…” (3).
Why is it that the literal blood of Jesus Christ had to be shed to forgive sin? Lewis says on its face it is a “very silly theory” (4), and “If God was prepared to let us off, why on earth did He not do so?” (5).
That is Christianity. That is what has to be believed. Any theories we build up as to how Christ’s death did all this are, in my view, quite secondary: mere plans or diagrams to be left alone if they do not help us, and, even if they do help us, not to be confused with the thing itself (6).
We may not fully comprehend why an innocent man—the Son of God—had to come to Earth, live a sinless life and be put to death on a Roman cross so that we, by a simple act of faith, can be born again, saved from our sins and from eternal damnation.
We have pictures: a Suffering Servant (Isaiah 53), and “the Lamb of God who takes away the sin of the world” (John 1:29), among others. We don’t need to get too deep into the weeds, understanding every nuance of God’s plan of salvation.
“Truly, truly, I say to you, he who believes has eternal life” (John 6:47). That is God’s formula. Scripture encourages us to investigate the matter, with the realization that we may not understand every last detail if it is the glory of God to conceal it.
References
1. Ernest Rutherford, John A. Ratcliffe, “Forty Years of Physics." In Needham, Joseph; Pagel, Walter (eds.). Background to Modern Science (New York: Cambridge University Press, 1938).
2. C. S. Lewis, Mere Christianity (Harper One, Harper Collins Publishers, New York, 2007), 55.
3. Lewis, Mere Christianity, 55.
4. Lewis, Mere Christianity, 56.
5. Lewis, Mere Christianity, 56.
6. Lewis, Mere Christianity, 55-56.
Gregory J. Rummo, M.S. M.B.A., is a Lecturer of Chemistry at Palm Beach Atlantic University and a DMin student at Knox Theological Seminary.
