God and Nature Winter 2023
By Jeffrey K. Greenberg
Colossians 1:17 says about Jesus that “He is before all things and He holds all things together.” Let’s think about what this means: Jesus is the glue, but He is also the craftsman who made the pieces. If we add this thought to the way John 1:1 harmonizes with one of the fundamental physical principles of our universe, the mass-energy equivalence expressed by Einstein’s famous equation E=mc^2 , we can discover a sweet expression of the faith-science relationship.
In the Beginning was the Big “E.” With a little “magic,” that energy source produces matter = m (as in mass). This statement is both a scientific and theological singularity. Through the E, or, in biblical terms, The Word, all things came into being. We are informed that the Godhead is Love. No one knows technically, scientifically, what love is, but we know about it via the Cross and the many ways we interact with Creation, especially with each other. We experience the reality of love, and Paul helps us with some useful definitions in 1 Corinthians Chapter 13. Love is the ultimate Energy!
Colossians 1:17 says about Jesus that “He is before all things and He holds all things together.” Let’s think about what this means: Jesus is the glue, but He is also the craftsman who made the pieces. If we add this thought to the way John 1:1 harmonizes with one of the fundamental physical principles of our universe, the mass-energy equivalence expressed by Einstein’s famous equation E=mc^2 , we can discover a sweet expression of the faith-science relationship.
In the Beginning was the Big “E.” With a little “magic,” that energy source produces matter = m (as in mass). This statement is both a scientific and theological singularity. Through the E, or, in biblical terms, The Word, all things came into being. We are informed that the Godhead is Love. No one knows technically, scientifically, what love is, but we know about it via the Cross and the many ways we interact with Creation, especially with each other. We experience the reality of love, and Paul helps us with some useful definitions in 1 Corinthians Chapter 13. Love is the ultimate Energy!
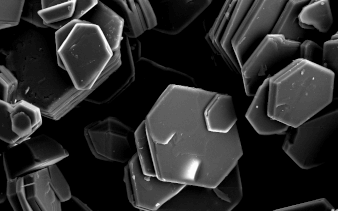
God’s works are mysterious, as shown by the electronic structure of copper. |
By the early 1900s, scientists had developed theories about chemical bonding. Although we treat these theories as reality, no one actually knows the details of how bonding occurs. It remains a mystery even while we gain more and more knowledge about atomic particles and many sub-atomic particles. With reference to light, we settle primarily on a dual definition including both wave and particle characteristics. The entire realm of Quantum Mechanics brings us into a new, ultimately elusive paradigm for how everything functions at the deeper levels of interaction. The great thing about powerful scientific theories is that they can provide effective, working concepts without our knowing all the intricacies.
In my introductory Physical Geology courses, I introduce the theme of minerals as basic building materials, with some foundational chemistry. Of course, we start with elements, those commodities born in star factories. These elements are collected via earthly reaction processes into associations through the attraction of bonding. I describe four fundamental bond types: ionic, covalent, metallic, and van der Walls. These four are demonstrated in various combinations of elements to form over 5000 naturally occurring mineral “species” (so far). I have found that students appreciate teaching that makes complex concepts applicable and less technical. In the context of teaching at a Christian college like Wheaton, it is also gratifying to see where scientific concepts can, without forcing, be tied to spiritual applications. The mystery of chemical bonding offers such an opportunity.
Ionic Bonds: These are best conceived as an association where two or more elements are attracted through the giving and taking of outer “shell” electrons that are less tightly held by atomic nuclei. A great example is halite, rock salt. NaCl has a simple cubic crystalline structure with sodium Na+1 (an electron giver) bonding to chlorine Cl-1 as the electron taker. Each atom is attempting to attain a stable chemical-atomic configuration of its electron energy shell. These ions binding together can be seen as a “one gets and one loses” situation. In human relationship terms, this may be analogous to the dysfunction of co-dependency. It turns out that ionic bonding can range in strength from moderate to quite weak, but never very strong. Halite is easily dissolved in the presence of water.
Covalent Bonds: Some chemical reactions produce an overlap of electron shells, such that there is a sharing of electron charge to compensate for an incomplete energy-level state. This bond type is most commonly found in organic chemistry (the chemistry of carbon atoms) and is the basis of biochemistry–the bonds of life. Here, the atomic nuclei are drawn closer. Atomic “maps,” derived from x-ray and other types of instrumental analysis, image spotty blobs that are closer together than in ionic associations. The overlap helps create a stronger bond, typified by diamond, in which carbon atoms are tightly joined in a cubic structure. Compare diamond with halite. The contrast is obvious. In spiritual terms, that sharing is like a marriage of mutual respect.
Metallic Bonds: God’s works are mysterious, as shown by the electronic structure of copper. Copper is a mineral species with a cubic crystal structure. In the case of some particularly metallic transition elements including copper, delocalized electrons are essentially fluid in movement within the structure rather than being tightly bound to individual atoms. This “cloud of electrons” imparts a weak but durable electrostatic charge to the entire crystal. The well-known metallic characteristics of being ductile, malleable, opaque, and good conductors of heat and electrical energy are due to that electron cloud. It is a wonder how much this contrasts with the first two bond types and how important it is as a gift for us in modern technological dependence.
Van der Walls Bonds: The last type of bonding is a kind of electrostatic attraction, the phenomenon that causes a balloon that has been rubbed against fabric to stick to a wall. This type of electron attraction is quite weak. Examples include the mica family of hydrous, layered silicates (phyllosilicates), and clay minerals with microscopic crystal grains. The structures are complex in that silicate (Si+O) substructures are bonded to each other as well as to various positively charged cations and water molecules. The various bonded combinations are stacked along one crystallographic direction, like a sandwich with different fillings. The internal bonds are mostly covalent with some ionic components. There is also a double occurrence of van der Waals bonds formed between some layers and less regularly on the surfaces of the crystals. The electrostatic nature of these bonds is very weak, leading to some intriguing properties in materials like clay. The larger-sized distances between layers allow an easy splitting of the crystals along that single plane of cleavage, between the layers. It’s like a thick book whose pages peel apart easily, but breaking apart the book from a plane 90 degrees away is nearly impossible.
On the surface of clay, mica, and other layered silicates, 90 degrees from the layering, there develops an electrostatic charge. This surface property, resulting in van der Waals forces, attracts materials with mostly positive charge. This includes water but also cations and even organic molecules. The capability to attract is well demonstrated by clay particles. Each clay crystal is exceedingly tiny, observable only with electron micrography, at 1/256mm or less in size. The surface charge of each and every crystal causes clay to aggregate with amazing stickiness. That property makes clay one of the Creator’s smallest wonders.
Clay can be used in many important applications, including medical, environmental, and aesthetic. Clay swallowed in an emulsion with liquid components has long been a treatment for digestive ailments. Microbes as well as some heavy metals and toxins get adsorbed onto the clay and then pass harmlessly through our systems. Clay aggregates have essentially 0% permeability with liquids, as long as the aggregate thickness is great enough. Landfill sites that contain toxic chemicals are legally mandated to have compacted clay liners of 3 feet in thickness to prevent pollutants from seeping down into the groundwater supply.
A certain amount of clay makes for good crop-growing soil and for stable excavation in building. Clay to about 25% in soil optimally aids good cohesion without being difficult to excavate. The best farm soils are “loams,” a combination of sand-sized, silt-sized, and clay-sized components. The right amount of clay interacts with water and dissolved materials in ion-exchange processes that make nutrients and water stick to the clay aggregates, facilitating healthy crops. Too much clay results in stunted-rotted roots; too little clay, in poor availability of essential plant foods.
I see in these stories about clay utility reflections of the Lord’s relationship in love for us. We have this stuff that requires some understanding. Once we do recognize how the Lord’s genius provides for life, it becomes our responsibility to steward the Creation at all levels. God holds all things together. He is the power of bonding. It is love and relationship that permeates the Lord’s total presence in Creation. He is not just in us, via the Holy Spirit, but His wisdom is in all of His Creation. A tree is not God; a mineral is not God; I am not God. But God’s imprint is everywhere. We do not worship Nature in Creation, but we worship the Creator and certainly should love the created as He does.
Jeff Greenberg retired from the faculty at Wheaton College, IL, after 33 years of teaching, mentoring, and doing research in geology. His greatest educational passion is in supervising students as junior practitioners for global development projects.
In my introductory Physical Geology courses, I introduce the theme of minerals as basic building materials, with some foundational chemistry. Of course, we start with elements, those commodities born in star factories. These elements are collected via earthly reaction processes into associations through the attraction of bonding. I describe four fundamental bond types: ionic, covalent, metallic, and van der Walls. These four are demonstrated in various combinations of elements to form over 5000 naturally occurring mineral “species” (so far). I have found that students appreciate teaching that makes complex concepts applicable and less technical. In the context of teaching at a Christian college like Wheaton, it is also gratifying to see where scientific concepts can, without forcing, be tied to spiritual applications. The mystery of chemical bonding offers such an opportunity.
Ionic Bonds: These are best conceived as an association where two or more elements are attracted through the giving and taking of outer “shell” electrons that are less tightly held by atomic nuclei. A great example is halite, rock salt. NaCl has a simple cubic crystalline structure with sodium Na+1 (an electron giver) bonding to chlorine Cl-1 as the electron taker. Each atom is attempting to attain a stable chemical-atomic configuration of its electron energy shell. These ions binding together can be seen as a “one gets and one loses” situation. In human relationship terms, this may be analogous to the dysfunction of co-dependency. It turns out that ionic bonding can range in strength from moderate to quite weak, but never very strong. Halite is easily dissolved in the presence of water.
Covalent Bonds: Some chemical reactions produce an overlap of electron shells, such that there is a sharing of electron charge to compensate for an incomplete energy-level state. This bond type is most commonly found in organic chemistry (the chemistry of carbon atoms) and is the basis of biochemistry–the bonds of life. Here, the atomic nuclei are drawn closer. Atomic “maps,” derived from x-ray and other types of instrumental analysis, image spotty blobs that are closer together than in ionic associations. The overlap helps create a stronger bond, typified by diamond, in which carbon atoms are tightly joined in a cubic structure. Compare diamond with halite. The contrast is obvious. In spiritual terms, that sharing is like a marriage of mutual respect.
Metallic Bonds: God’s works are mysterious, as shown by the electronic structure of copper. Copper is a mineral species with a cubic crystal structure. In the case of some particularly metallic transition elements including copper, delocalized electrons are essentially fluid in movement within the structure rather than being tightly bound to individual atoms. This “cloud of electrons” imparts a weak but durable electrostatic charge to the entire crystal. The well-known metallic characteristics of being ductile, malleable, opaque, and good conductors of heat and electrical energy are due to that electron cloud. It is a wonder how much this contrasts with the first two bond types and how important it is as a gift for us in modern technological dependence.
Van der Walls Bonds: The last type of bonding is a kind of electrostatic attraction, the phenomenon that causes a balloon that has been rubbed against fabric to stick to a wall. This type of electron attraction is quite weak. Examples include the mica family of hydrous, layered silicates (phyllosilicates), and clay minerals with microscopic crystal grains. The structures are complex in that silicate (Si+O) substructures are bonded to each other as well as to various positively charged cations and water molecules. The various bonded combinations are stacked along one crystallographic direction, like a sandwich with different fillings. The internal bonds are mostly covalent with some ionic components. There is also a double occurrence of van der Waals bonds formed between some layers and less regularly on the surfaces of the crystals. The electrostatic nature of these bonds is very weak, leading to some intriguing properties in materials like clay. The larger-sized distances between layers allow an easy splitting of the crystals along that single plane of cleavage, between the layers. It’s like a thick book whose pages peel apart easily, but breaking apart the book from a plane 90 degrees away is nearly impossible.
On the surface of clay, mica, and other layered silicates, 90 degrees from the layering, there develops an electrostatic charge. This surface property, resulting in van der Waals forces, attracts materials with mostly positive charge. This includes water but also cations and even organic molecules. The capability to attract is well demonstrated by clay particles. Each clay crystal is exceedingly tiny, observable only with electron micrography, at 1/256mm or less in size. The surface charge of each and every crystal causes clay to aggregate with amazing stickiness. That property makes clay one of the Creator’s smallest wonders.
Clay can be used in many important applications, including medical, environmental, and aesthetic. Clay swallowed in an emulsion with liquid components has long been a treatment for digestive ailments. Microbes as well as some heavy metals and toxins get adsorbed onto the clay and then pass harmlessly through our systems. Clay aggregates have essentially 0% permeability with liquids, as long as the aggregate thickness is great enough. Landfill sites that contain toxic chemicals are legally mandated to have compacted clay liners of 3 feet in thickness to prevent pollutants from seeping down into the groundwater supply.
A certain amount of clay makes for good crop-growing soil and for stable excavation in building. Clay to about 25% in soil optimally aids good cohesion without being difficult to excavate. The best farm soils are “loams,” a combination of sand-sized, silt-sized, and clay-sized components. The right amount of clay interacts with water and dissolved materials in ion-exchange processes that make nutrients and water stick to the clay aggregates, facilitating healthy crops. Too much clay results in stunted-rotted roots; too little clay, in poor availability of essential plant foods.
I see in these stories about clay utility reflections of the Lord’s relationship in love for us. We have this stuff that requires some understanding. Once we do recognize how the Lord’s genius provides for life, it becomes our responsibility to steward the Creation at all levels. God holds all things together. He is the power of bonding. It is love and relationship that permeates the Lord’s total presence in Creation. He is not just in us, via the Holy Spirit, but His wisdom is in all of His Creation. A tree is not God; a mineral is not God; I am not God. But God’s imprint is everywhere. We do not worship Nature in Creation, but we worship the Creator and certainly should love the created as He does.
Jeff Greenberg retired from the faculty at Wheaton College, IL, after 33 years of teaching, mentoring, and doing research in geology. His greatest educational passion is in supervising students as junior practitioners for global development projects.
